Cleanroom Operations
Background
Cleanrooms are defined as a room or suite of rooms, in which the concentration of airborne particles is maintained within established parameters and where other factors are controlled to within specified limits. These rooms are designed to provide control of environmental factors including:
- - Viable and non-viable airborneparticles
- - Air flow patterns
- - Temperature and humidity
- - Air pressure differential
- - Containment of hazardousaerosols

Applications include the manufacture of sterile and non-sterile pharmaceutical and biotech products, medical devices, and implants. These rooms are also used to manufacture sensitive electronics. However, the requirements for these latter applications are not the objective of this article. GMP requirements from the different Boards of Health, including the US Food and Drug Administration (FDA), for sterile pharmaceutical, biotech, medical devices and implants require the manufacture of these products are performed in clean environments that meet the requirements of standards such as ISO EN146441.
- FDA Guideline Sterile DrugProducts Produced by AsepticProcessing2
- European Union (EU) GMPAnnex 13 Manufacture of SterileMedicinal Products
Compliance Requirements:
- ISO EN14644-1, -2
- International Society for Pharmaceutical Engineering (ISPE4)
Other Helpful Documents:
Air Handling Requirements
Room air should be supplied by an external air conditioning system, preferably dedicated to the facility. Partial recirculation of room air is appropriate and this allows for optimal energy utilization. Sufficient fresh air should be supplied in accordance with ventilation codes, to balance exhaust air and to maintain specified pressures. Unless, otherwise specified, typical temperature range for this kind of room is within the range of 16 to 19 °C and relative humidity of approximately 50% is maintained. The type of equipment and number of people in the room may dictate where in the range you need to be to assure that during production the operations area is maintained at the right temperature and humidity levels.
Only HEPA filtered air should enter the cleanroom and the gowning areas. These modules are available in fan assisted with fan speed control and should operate at a velocity of 90 ± 10 fpm or 0.45 ± 0.05 m/s. The location of the HEPA filters and air return grilles should create air movement from the designated ‘clean zone’ to the ‘less clean’ zones. Return air grilles should be at a lower level to aid in laminar flow requirements.
Air supply to the cleanroom should provide a room air change rate of >20 per hour. Air cleanliness will be enhanced by higher air change rates. When the doors are opened the supply air volume should maintain an outward flow of air.
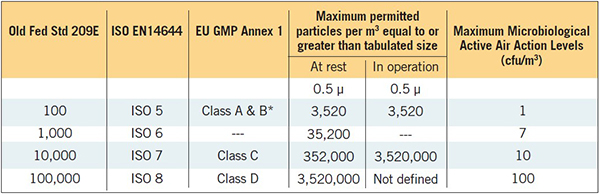
*Requirements for particle counts during operation are different from class A, 35,200 particles at 0.5 μ
Sources of Contamination
In order to control contamination, operators and those in charge of a cleanroom need to be cognizant of sources of contamination. These include:
- Facilities: walls, floors andceilings; paint and coatings; spillsand leaks
- People: skin flakes and oil;cosmetics and perfume; spittle;clothing debris (lint, fibers, etc.);hair
- Tool-generated: friction andwear particles; lubricants andemissions; vibrations; brooms,mops, and dusters
- Fluids: particulates floating in air;bacteria, organics, and moisture;floor finishes or coatings;cleaning chemicals; plasticizers(outgasses); water
- Product-generated: glass flakes;cleanroom debris; aluminumparticles from vial caps
Key Elements of Contamination Control
Cleanroom Architecture – Cleanrooms are designed to achieve and maintain an airflow in which essentially the entire body of air within a confined area moves with uniform velocity along parallel flow lines. This air flow is called laminar flow. The more restricted the air flow, the more turbulence and this can cause undesireable particle movement.
Filtration – In addition to theHEPA filters commonly used incleanrooms, there are a numberof other filtration mechanismsused to remove particles fromgases and liquids used in themanufacture of pharmaceuticalproducts. These filters areessential for providing effectivecontamination control.
Cleanroom Garments – Therequirements for cleanroomgarments will vary from locationto location. It is importantto know the local cleanroomgarment requirements. Gloves,face masks and head coversare standard in nearly everycleanroom environment as wellas coveralls.
Personnel - There are bothphysical and psychologicalconcerns when humansare present in cleanrooms.Physical behavior like fastmotion and horseplay canincrease contamination.Psychological concerns likecomfort, claustrophobia, strongodors, and workplace attitudeare important. Below areseveral ways people producecontamination:
- Behavior-- Rate of movement, sneezing and coughing
- Attitude-- Work habits andcommunication betweenworkers
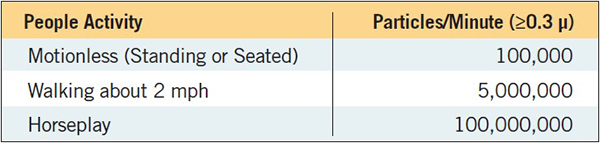
People are a major source of contamination in the cleanroom (Table 1). Notice the number of particles produced per minute during these activities.
Cleanroom Classifications
Cleanroom Classifications as specified by different standards are shown in Table 2. Non-sterile products are typically produced in an ISO 8 or EU Class D environment. Sterile products are required to be filled and stoppered in an ISO 5 or EU Class A environment.
Cleanroom Cleaning
As an example, here are some recommended procedures for cleaning a Class C or ISO 7 Area cleanroom. These procedures are simply guidelines, not standards or rules. It is important to review all cleaning procedures to be used in a cleanroom with responsible management. A detailed cleaning schedule should be prepared for every cleanroom.
Procedure
Good housekeeping and maintenance of the cleanroom and the associated restricted areas are essential to assure quality. Cleaning of an active cleanroom should be performed daily. However, if the room is not used daily, a different schedule may be implemented, but it should be cleaned after every use. Improper cleaning of the cleanroom can lead to contamination and compromise product quality. Proper selection of equipment, cleaning agents and cleaning materials is important for proper cleaning. Only products that have proven cleanroom performance records should be considered for use. These products should be listed in appropriate policies or procedures and all vendors should be informed about the strict policies of how products are qualified. All procedures should be strictly enforced. Below are some examples of how to organize cleanroom cleaning. These are guidelines for preparing work procedures and schedules. Local requirements must be included in any cleaning program.
Equipment and Supplies – all supplies must meet the Class C or ISO 7 Area minimum requirements
- Cleaning and disinfecting solutions
- Cleanroom mops
- Cleanroom vacuum cleaner(if allowed)
- Cleanroom wipers
- Cleanroom mop bucket andwringer
Cleaning Tasks – frequency may vary depending upon local requirements
- Cleaning of all work surfaces inthe controlled environment
- Vacuuming (if allowed) of thefloors and work surfaces
- Emptying of appropriate trashand waste receptacles
- Cleaning of the doors, doorframes and lockers in the pre-staging area and gowning areasusing the approved cleaningsolution
- Mop gowning area and cleanroomfloors
General Cleanroom Requirements
Here is a list of general requirements recommended as a minimum for the successful operation of a cleanroom. All cleanroom personnel should be aware and follow these requirements at all times.
- All personal items such as keys,watches, rings, matches, lightersand cigarettes should be storedin the personal locker outside thegowning room.
- Valuable personal Items suchas wallets may be permitted inthe cleanroom provided they arenever removed from beneath thecleanroom garments.
- No eating, smoking or gumchewing is allowed inside thecleanroom.
- Only garments approved forthe cleanroom should be wornwhen entering.
- No cosmetics shall be worn inthe cleanrooms. This includes:rouge, lipstick, eye shadow,eyebrow pencil, mascara, eyeliner, false eye lashes, fingernailpolish, hair spray, mousse, orthe heavy use of aerosols, aftershaves and perfumes.
- Only approved cleanroom paper shall be allowed in thecleanroom.
- Gloves should not be allowedto touch any item or surfacethat has not been thoroughlycleaned.
- Only approved gloves, pliers,tweezers should be used tohandle product.
- All tools, containers and fixturesused in the cleaning processshould be cleaned to thesame degree as the cleanroomsurfaces.
- No tool should be allowed torest on the surface of a benchor table. It should be placed ona cleanroom wiper.
- Only cleanroom approvedwipers are allowed to be used.The wipers must be approvedfor the class of cleanroom beingcleaned.
- All equipment, materials andcontainers introduced into asterile facility must be subjectedto stringent sterilization prior toentrance.
- No one who is physically ill,especially with respiratory orstomach disorders, may enter asterile room.
Definitions
HEPA Filter - High Efficiency Particulate Air Filter
Viable - a particle capable of living, developing, or germinating under favorable conditions, i.e., bacteria.
Non-viable - typically dust or liquid particles
Airborne - carried by or through the air
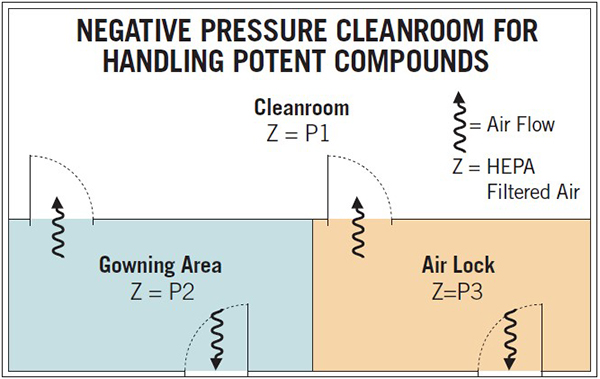
Cleanrooms for Handling Potent Compounds For handling potent compounds, i.e., toxic materials, in addition to the requirements described for a cleanroom, precautions need to be taken to avoid spreading the toxic material to adjacent areas. This may be achieved by maintaining a negative pressure differential between the cleanroom and adjacent area so any hazardous powder or aerosol is contained within the cleanroom, i.e., P3 ~ P2 > P1 (Figure 1). This is accomplished by designing the facility with HEPA filtered incoming air and providing HEPA filtration at the exhaust. Also providing a higher flow through the exhaust filters to assure proper flow, pressure differential and sufficient air changes in order to meet the required room classification; this setup does not allow air from the cleanroom to enter into adjacent areas. Additionally, by providing localized exhaust or incorporating isolators onto manufacturing equipment, the control of aerosols and dust from handling powders is increased. Note that personnel enter the cleanroom through the gowning area whereas equipment and materials are brought through the air lock.
References
- ISO EN 14644-1, -2, -5, -9International Standards Organization Cleanroom Standards
- FDA Guidance for Industry –Sterile Drug Products produced by Aseptic Processing – CGMP September 2004
- EU GMP Annex 1- Manufacture of Sterile Medicinal Products
- ISPE – International Societyfor Pharmaceutical Engineers



